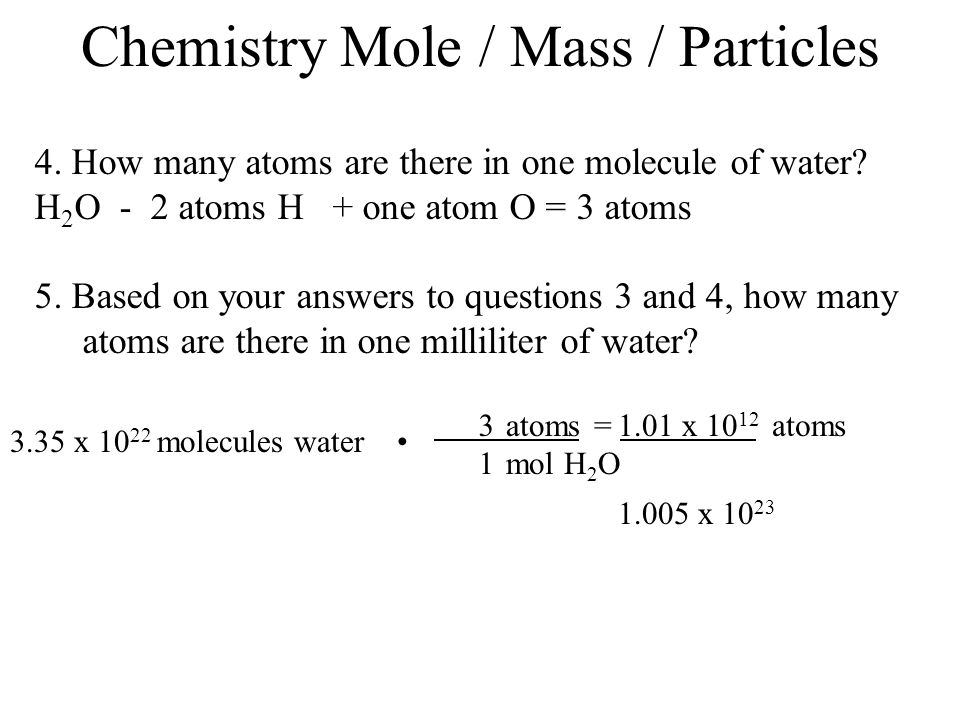
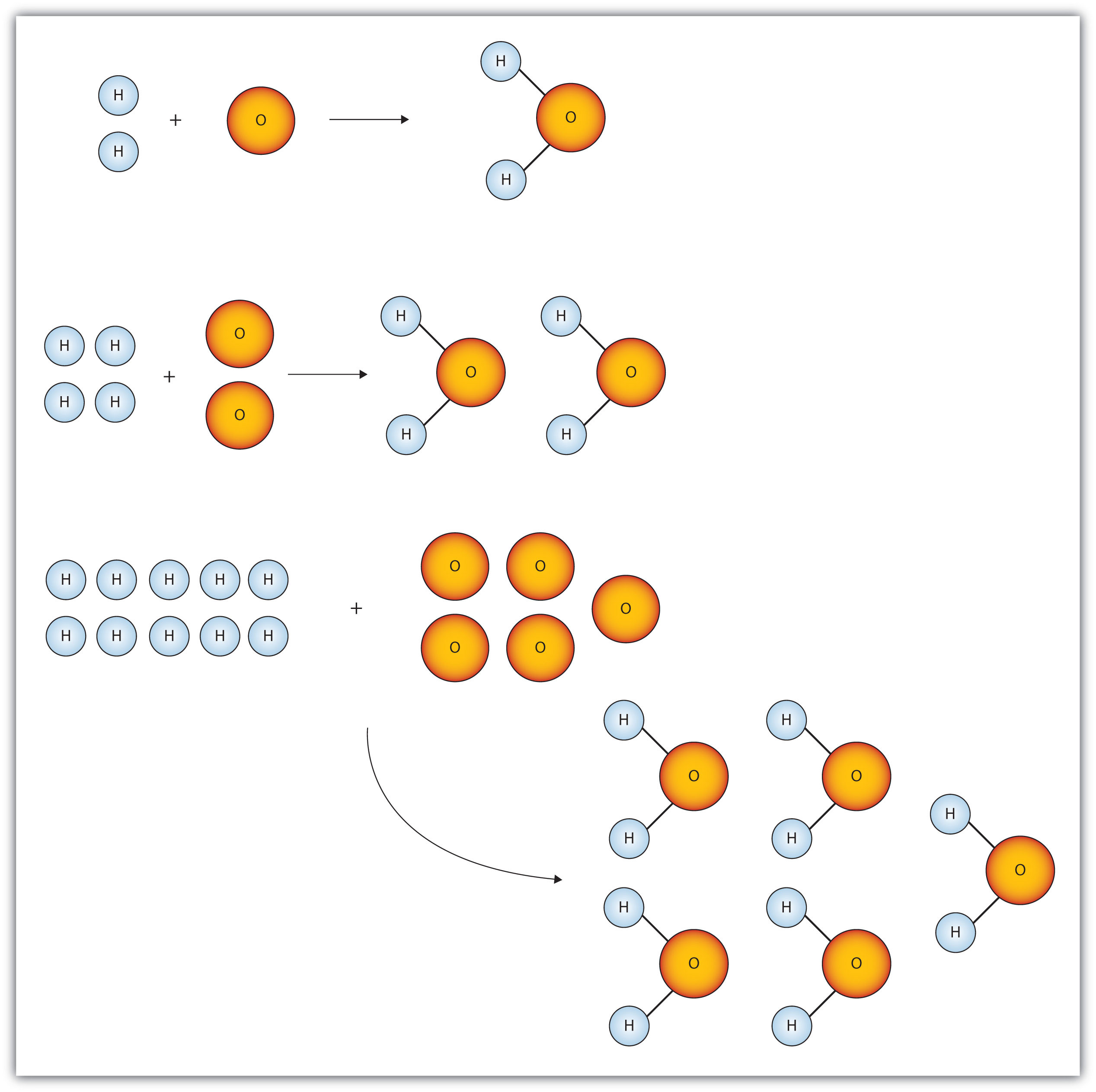
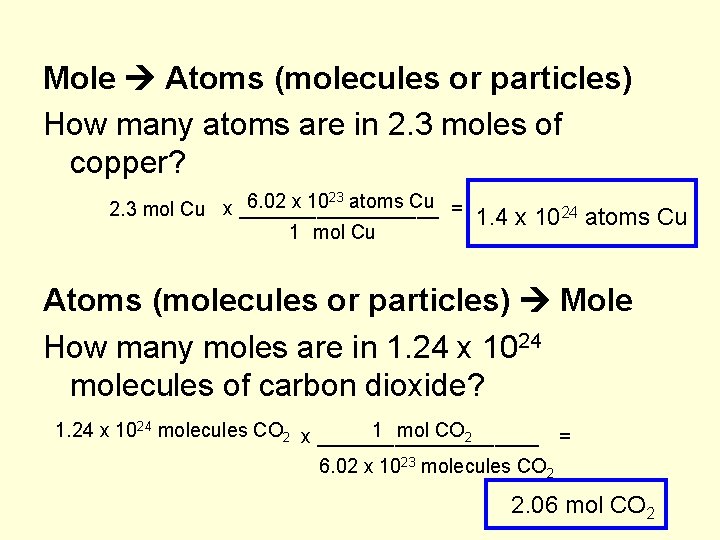
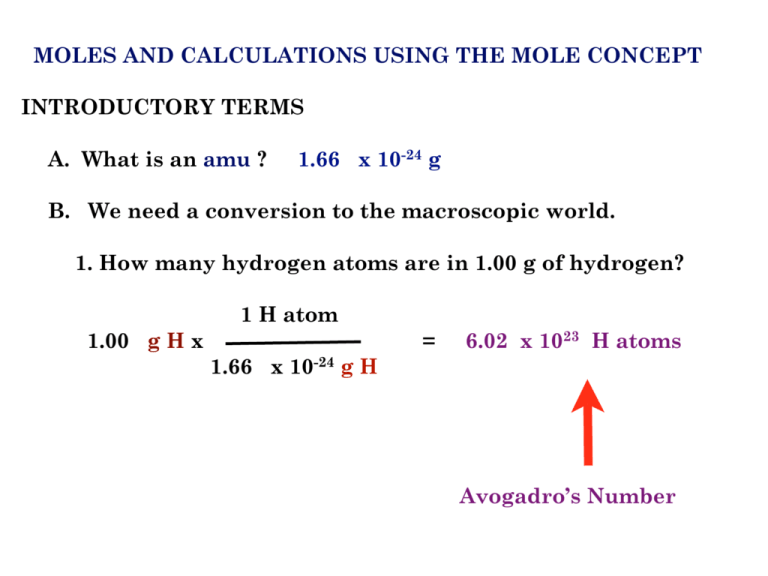
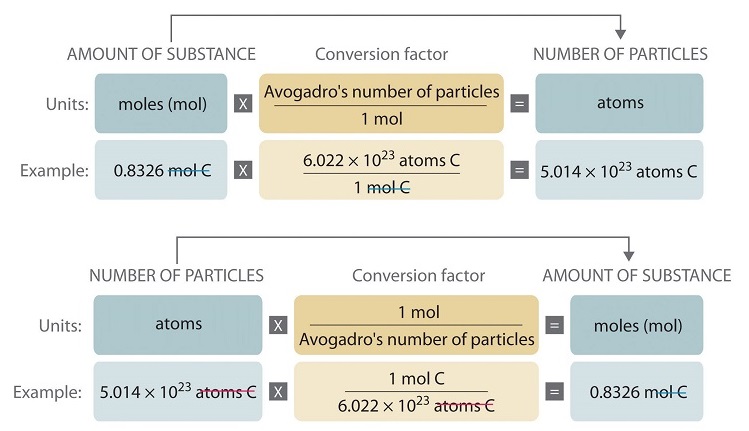
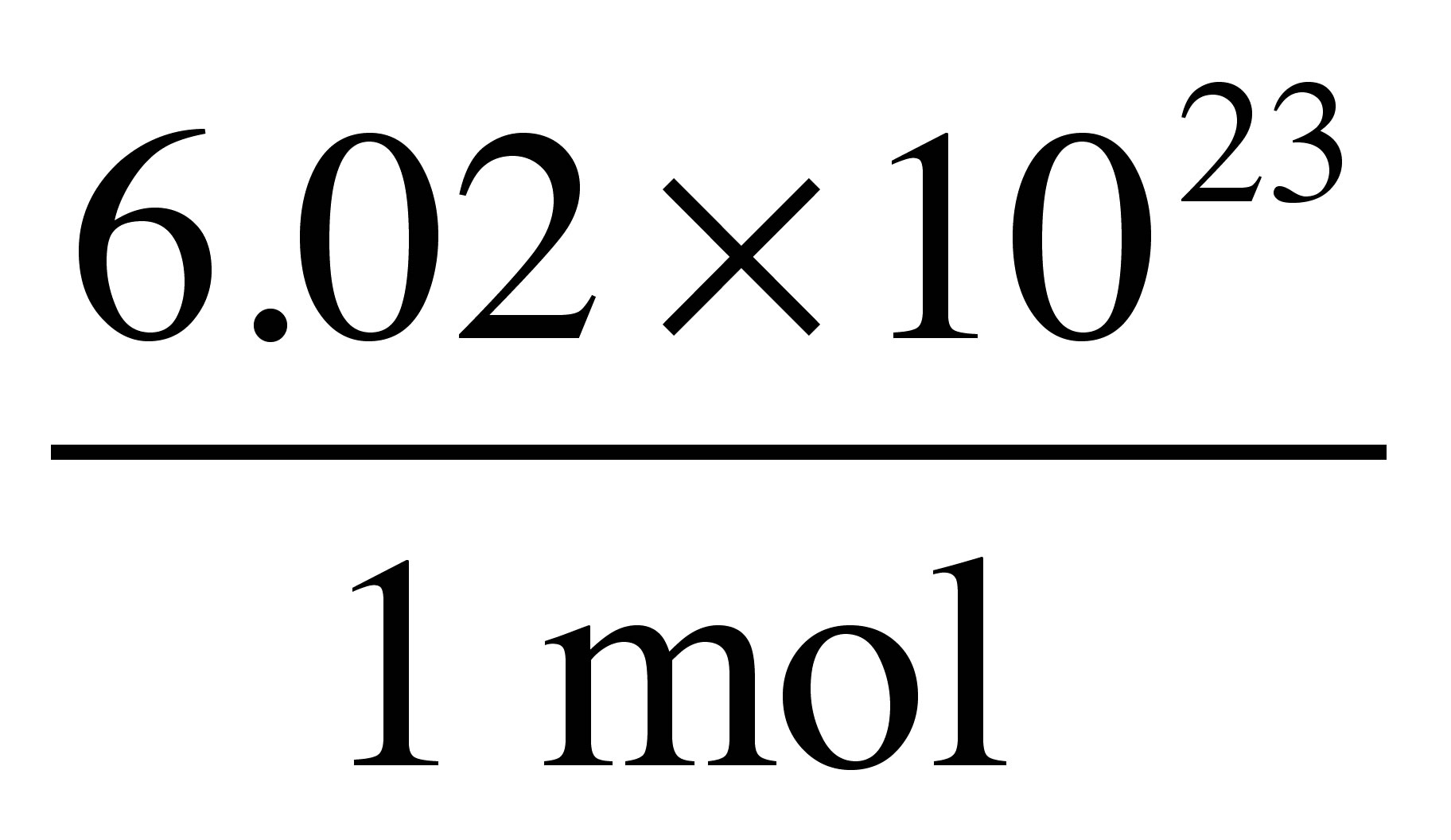The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download
The Mole Standards 1 dozen = 1 gross = 1 ream = 1 mole = x There are exactly 12 grams of carbon-12 in one mole of carbon ppt download