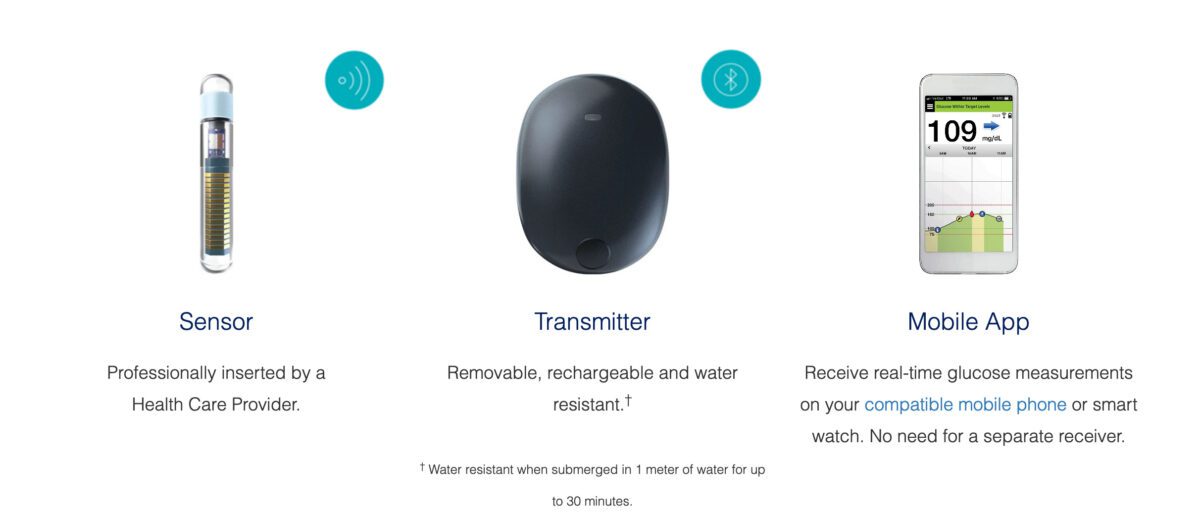
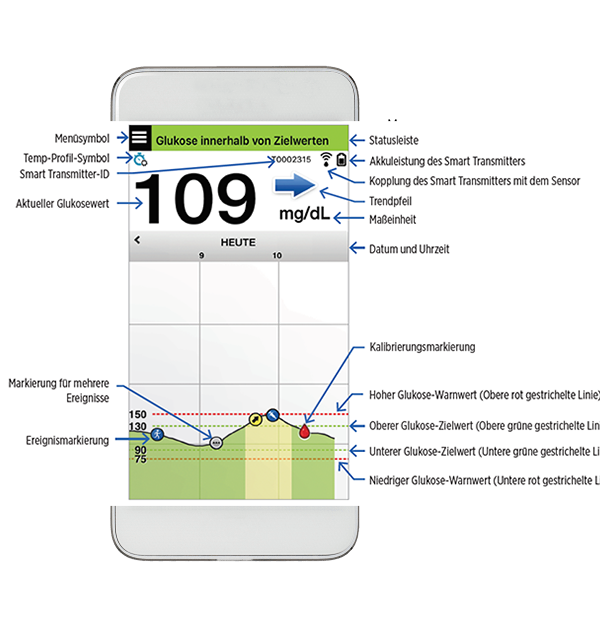
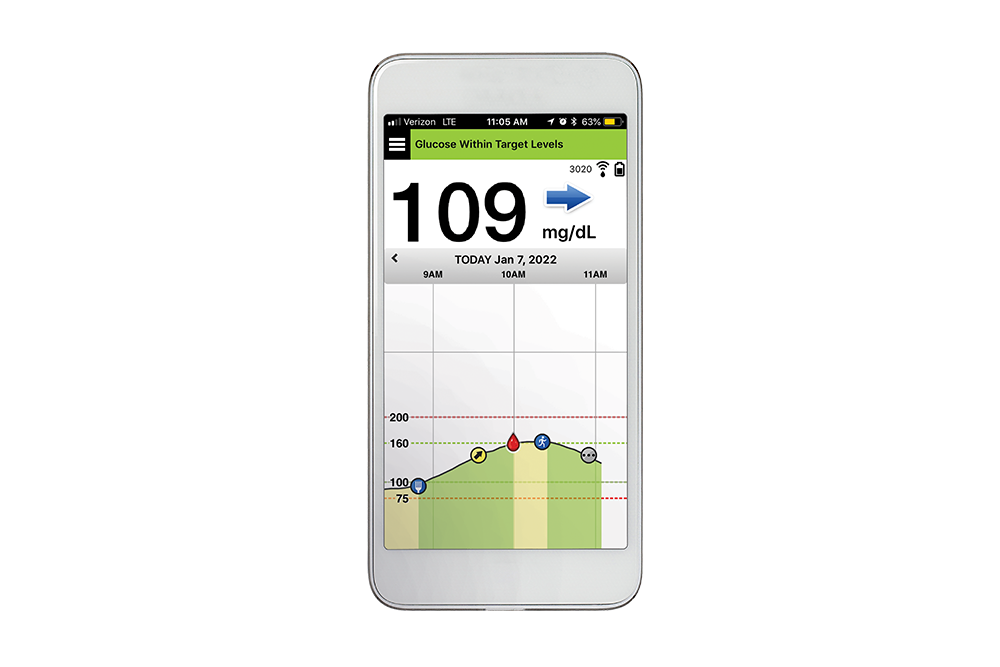
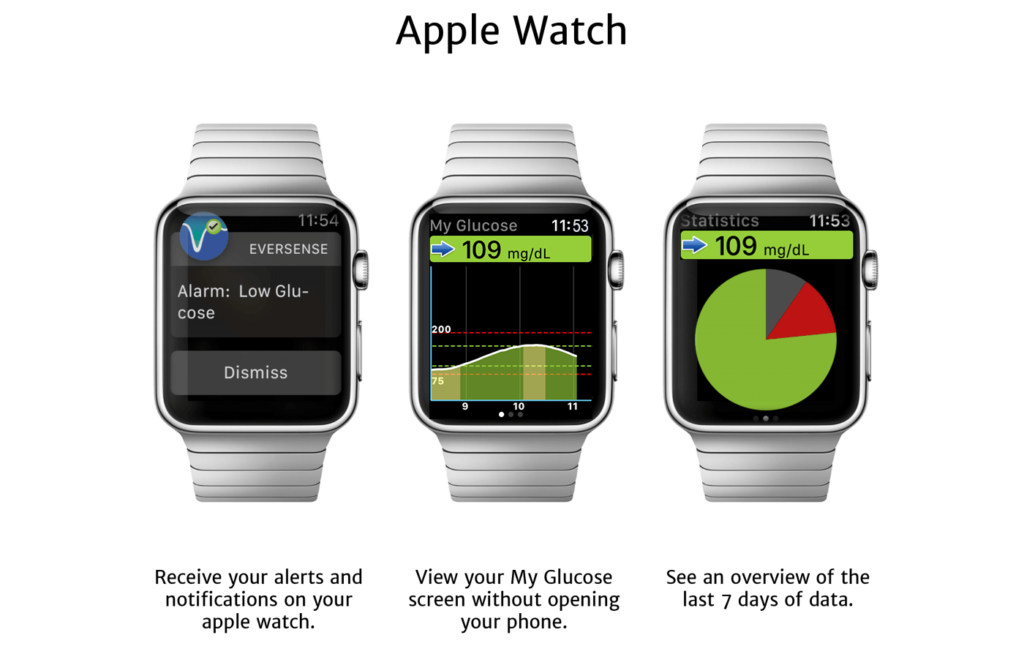
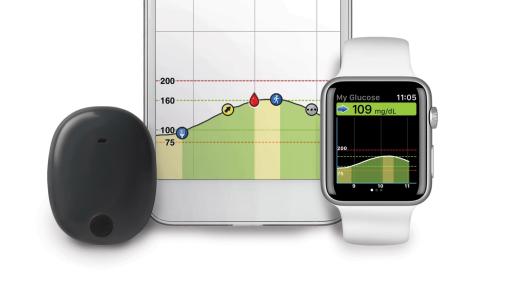
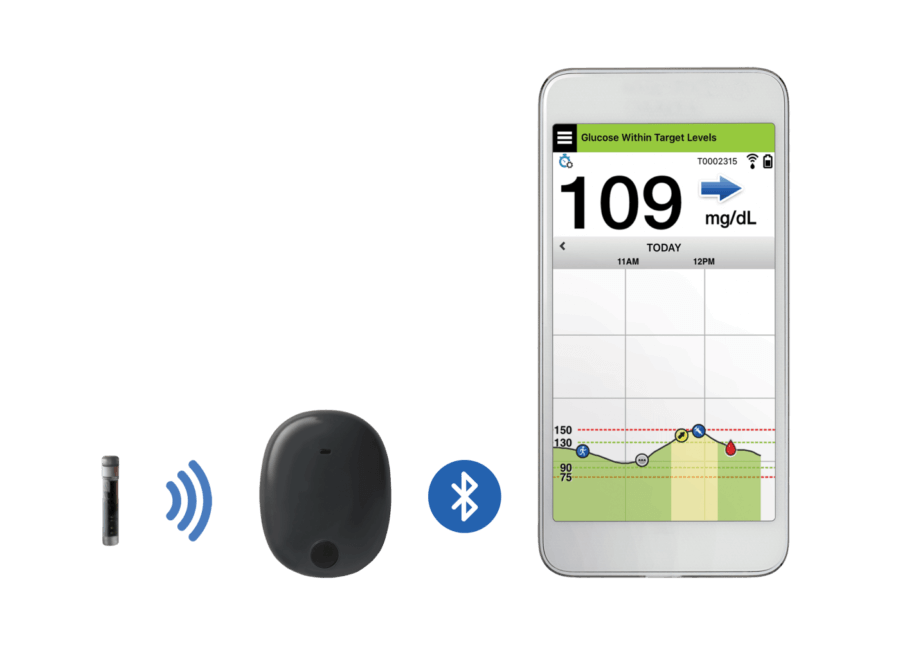
FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News