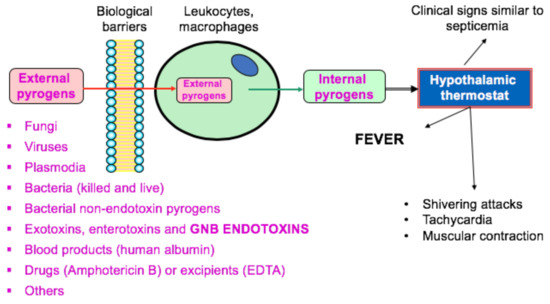
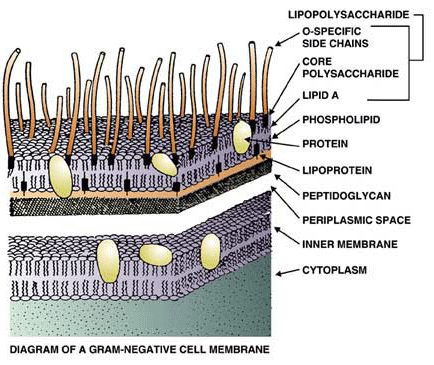
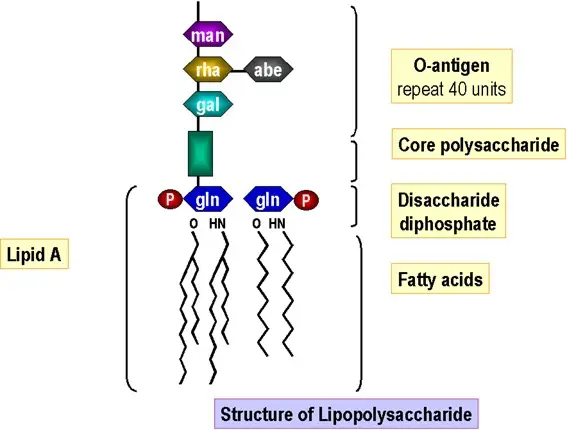
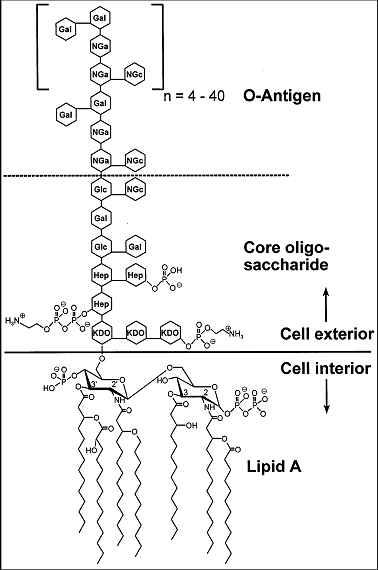
Puerarin exerts antipyretic effect on lipopolysaccharide-induced fever in rats involving inhibition of pyrogen production from macrophages - ScienceDirect

Pyrogen testing revisited on occasion of the 25th anniversary of the whole blood monocyte activation test | ALTEX - Alternatives to animal experimentation
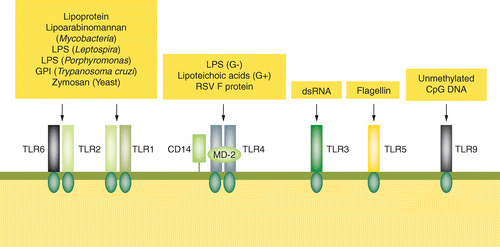
![Pyrogen Detection: Endotoxin, Peptidoglycan and β-D-Glucan | [Pharma Manufacturing & QC] | Laboratory Chemicals-FUJIFILM Wako Chemicals Europe GmbH Pyrogen Detection: Endotoxin, Peptidoglycan and β-D-Glucan | [Pharma Manufacturing & QC] | Laboratory Chemicals-FUJIFILM Wako Chemicals Europe GmbH](https://labchem-wako.fujifilm.com/europe/category/images/Pyrogen_Detection_img03.jpg)
Pyrogen Detection: Endotoxin, Peptidoglycan and β-D-Glucan | [Pharma Manufacturing & QC] | Laboratory Chemicals-FUJIFILM Wako Chemicals Europe GmbH
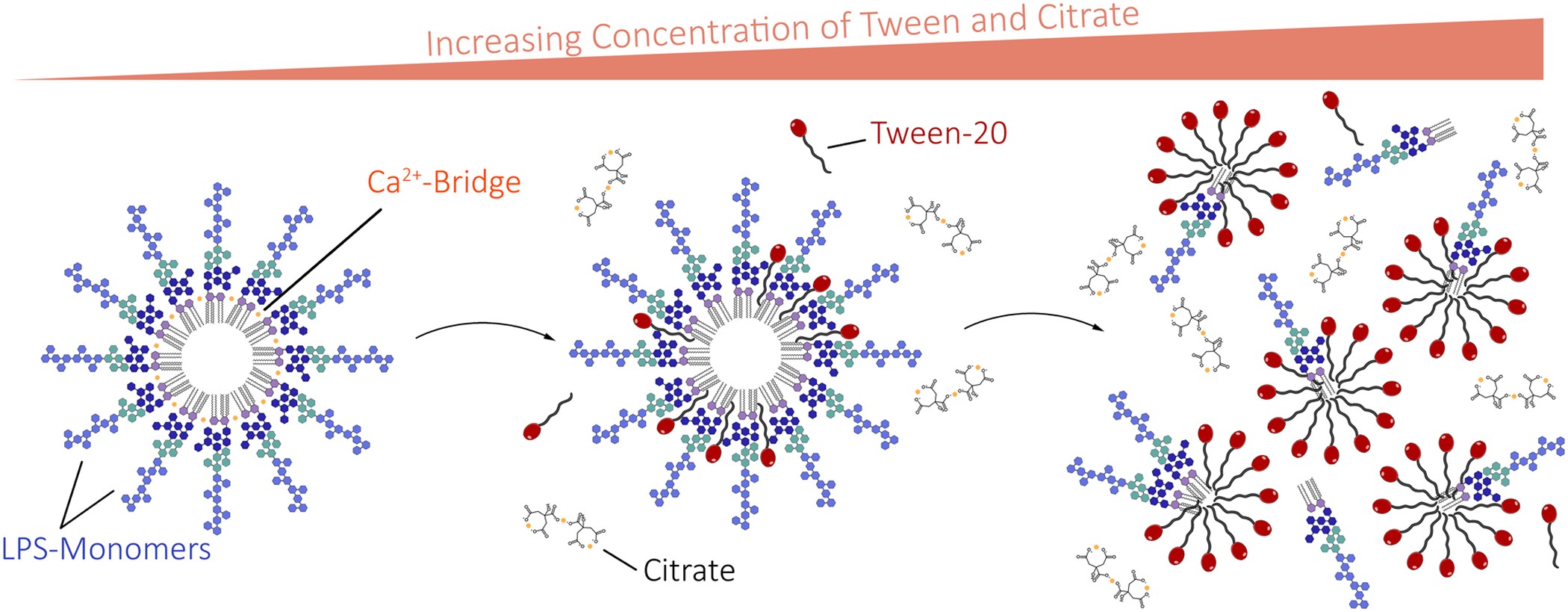
An in vitro study on factors affecting endotoxin neutralization in human plasma using the Limulus amebocyte lysate test | Scientific Reports
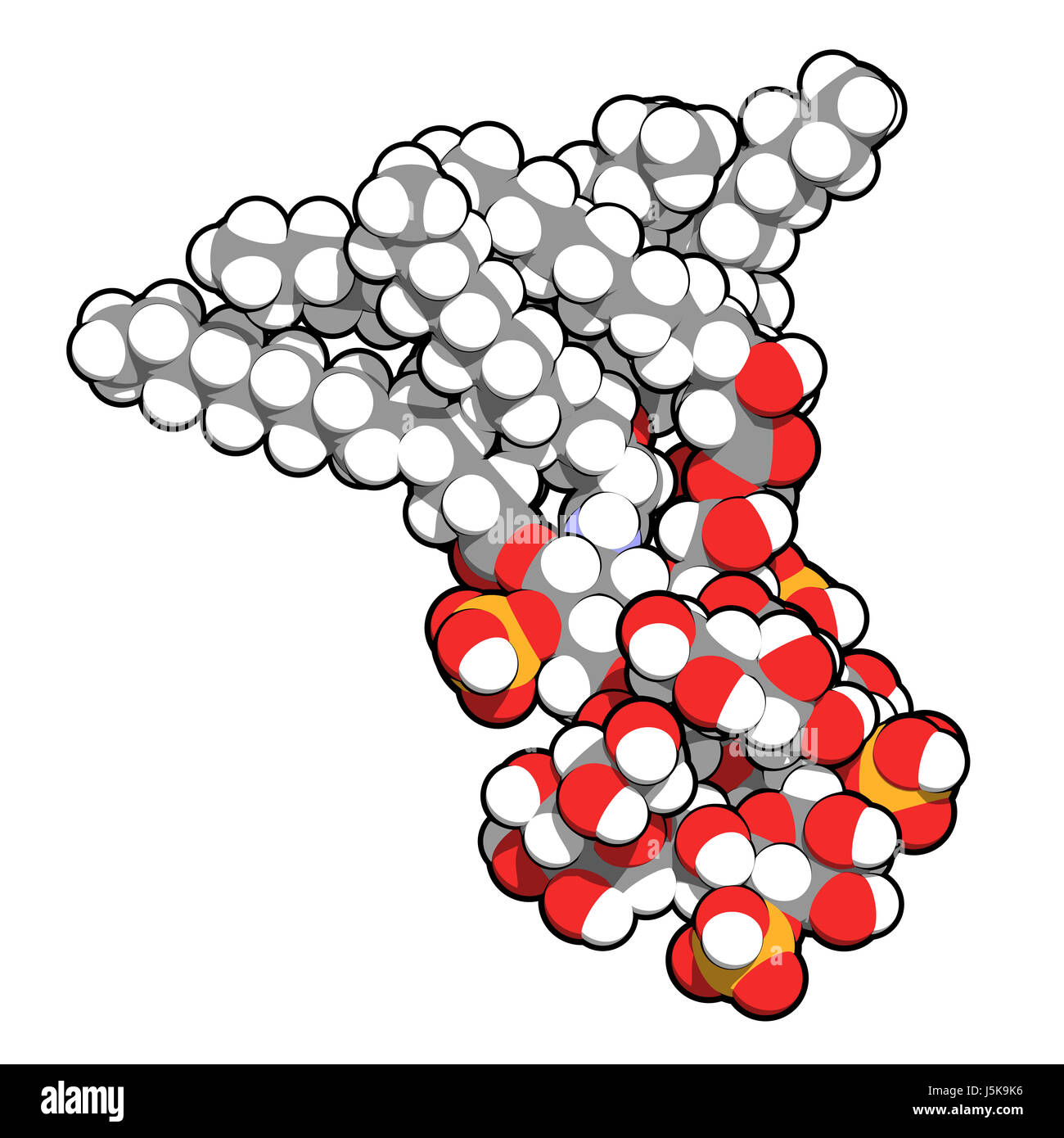
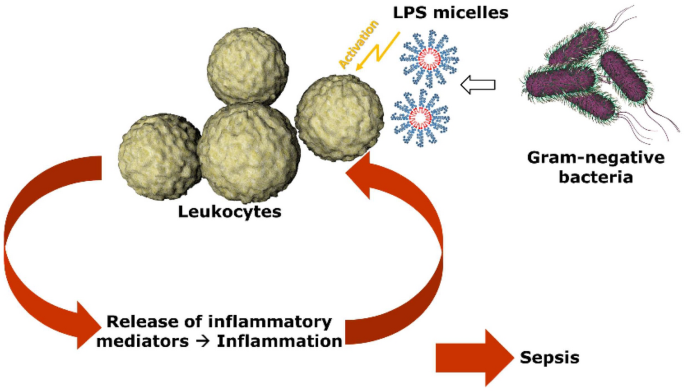
Lipopolysaccharide (LPS, lipid A and inner core fragment) endotoxin molecule from E. coli. 3D rendering based on protein data bank entry 3fxi Stock Photo - Alamy
Pyrogen Testing Revisited on Occasion of the 25th Anniversary of the Whole Blood Monocyte Activation Test. - Document - Gale OneFile: Health and Medicine

Soluble β-(1,3)-glucans enhance LPS-induced response in the monocyte activation test, but inhibit LPS-mediated febrile response in rabbits: Implications for pyrogenicity tests - ScienceDirect

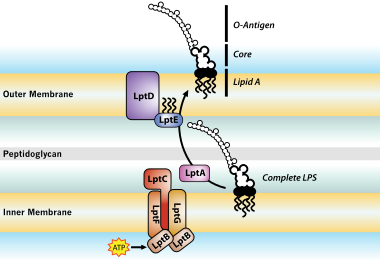
Https://Pyrogen-free.creativebiomart.net/Products/Lps-and-derivatives.html "Endotoxin (LPS) preparations are widely used to study the structure, metabolism, immunology, and biosynthesis of LPS. LPS is also used to induce the synthesis and secretion of ...

Functional Challenges for Alternative Bacterial Endotoxins Tests Part 4: Beyond Recombinant Reagents Introduction | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology