Calculate the 'spin only' magnetic moment of M2+ (aq) ion (Z = 27). - Sarthaks eConnect | Largest Online Education Community

Magnetic moment of `Cr^(+2)(Z=24),Mn^(+2)(Z=25)` and `Fe^(2+) (Z=26)` are x,y,z. they are in order - YouTube

Which of the following has maximum magnetic moment 1) Fe3+ 2) Mn2+ 3) Mn3+ 4) V3+ - Chemistry - - 14981787 | Meritnation.com

Among V(Z = 23) , Cr(Z = 24) , Mn(Z = 25) and Fe(Z = 26) , which will have the highest magnetic moment?

The correct order of spin only magnetic moment (inBM) for Mn^{2+} Cr^{2+} and Ti^{2+} ions is(a} Mn^{2+}>Ti^{2+}>Cr^{2+} (b) Ti^{2+}>Cr^{2+}>Mn^{2+}(c) Mn^{2+}>Cr^{2+}>T^{2+} (d} Cr^{2+}>Ti^{2+}>Mn^{2+} | Snapsolve

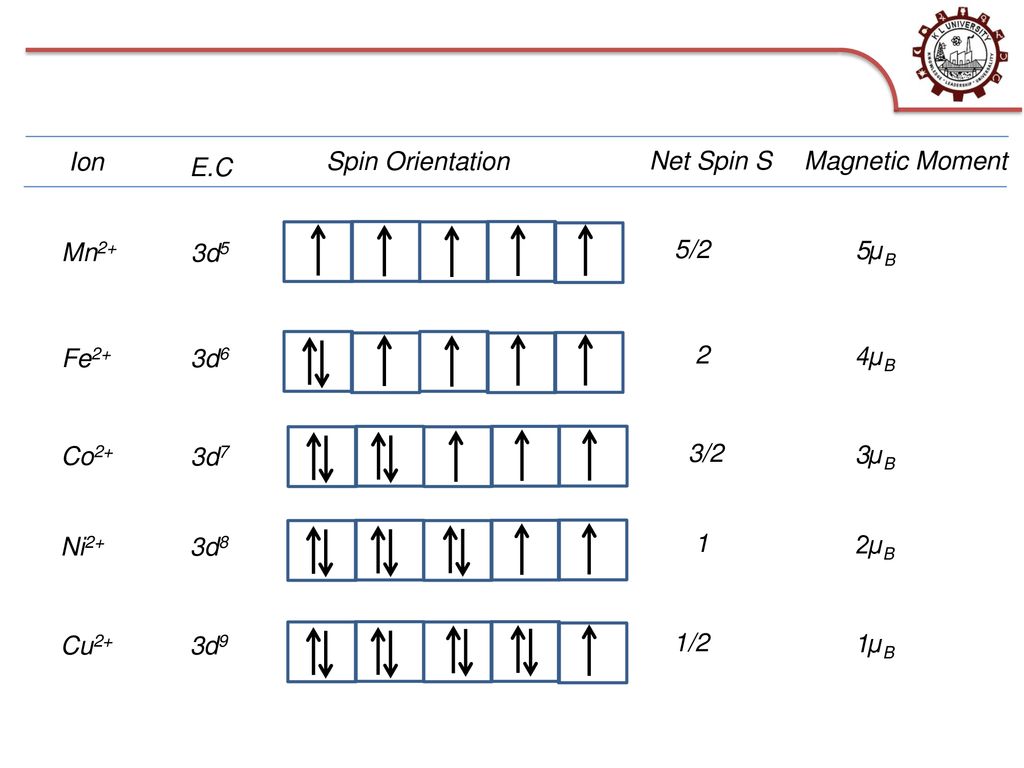
How many unpaired electrons are present in Mn^(2+) ion ? How do they influence magnetic behaviour of Mn^(2+) ion?

In the fallowing reaction: Cr2O7^2 - (aq) + SO3^2 - (aq) + 8H^+→ 2Cr^3 + + SO4^2 - + H2O the stoichiometric coefficient of SO3^2 - is:
![SOLVED:Calculate the spin-only magnetic moment (Us for the following metal ion. [Note that on the exam you will have to show your work]: High spin Mn2+ SOLVED:Calculate the spin-only magnetic moment (Us for the following metal ion. [Note that on the exam you will have to show your work]: High spin Mn2+](https://cdn.numerade.com/ask_images/bfe7cff006004e7aa567a2395d418232.jpg)
![Solved Mn4+ = [Ar]3d3 Mn2+ = [Ar] 3d5 Mn2+ (HS) Mn2+ (LS) eg | Chegg.com Solved Mn4+ = [Ar]3d3 Mn2+ = [Ar] 3d5 Mn2+ (HS) Mn2+ (LS) eg | Chegg.com](https://media.cheggcdn.com/media/8e7/8e73120c-3a2f-4a30-aaa4-f1ec151322c6/phpFuSNeX)