Sodium acetate buffer 5.2 +/- 0.1 25°C, for molecular biology, 3M, 0.2 μm filtered | 126-96-5 | Sigma-Aldrich

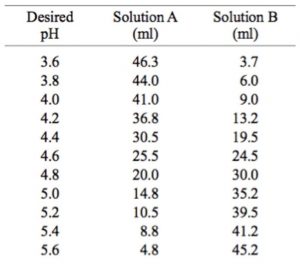
A buffer solution is prepared by mixing `10ml` of `1.0 M` acetic acid & `20 ml` of `0.5 M` - YouTube

Effect of pH (in Na-acetate buffer, 50 mM, at pH 4.0-6.0 and in sodium... | Download Scientific Diagram
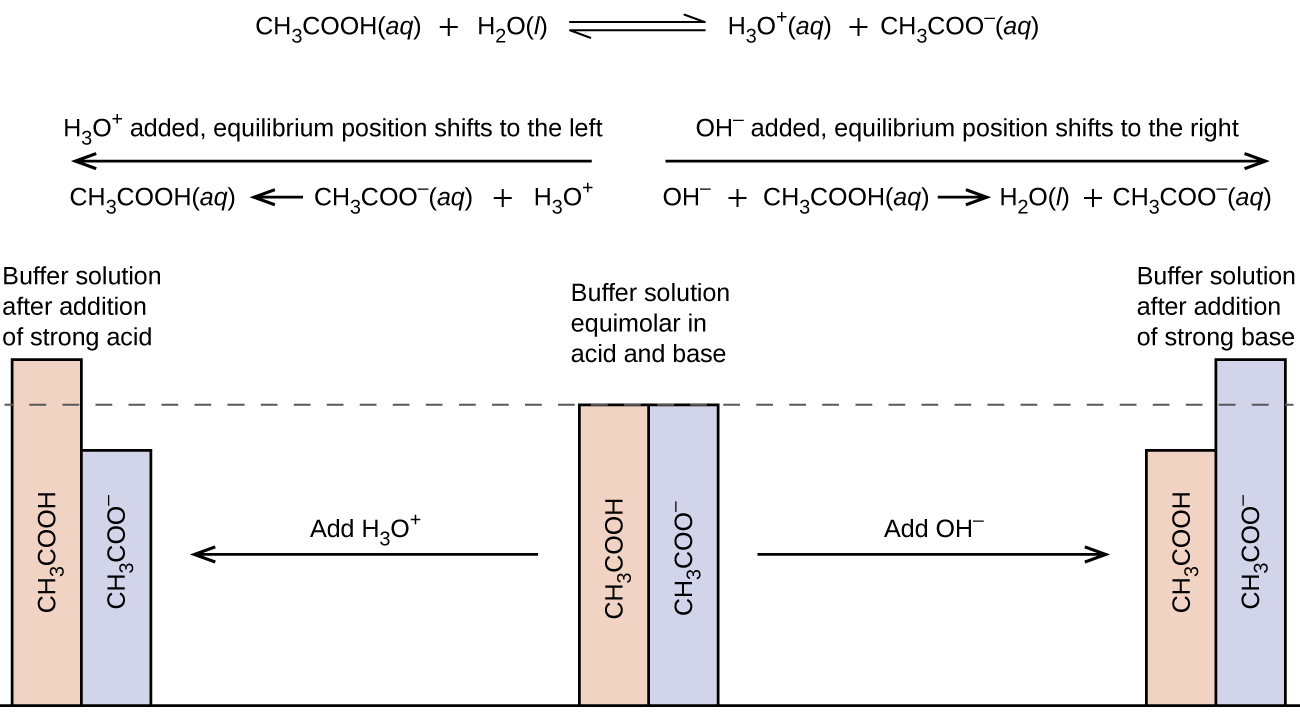
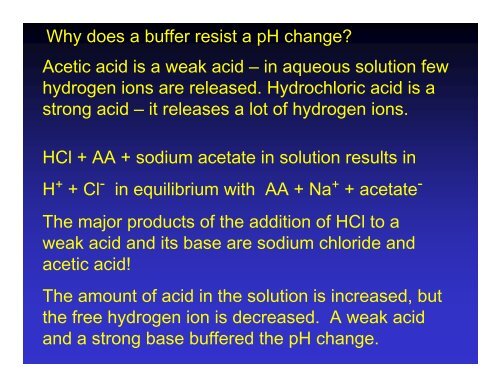
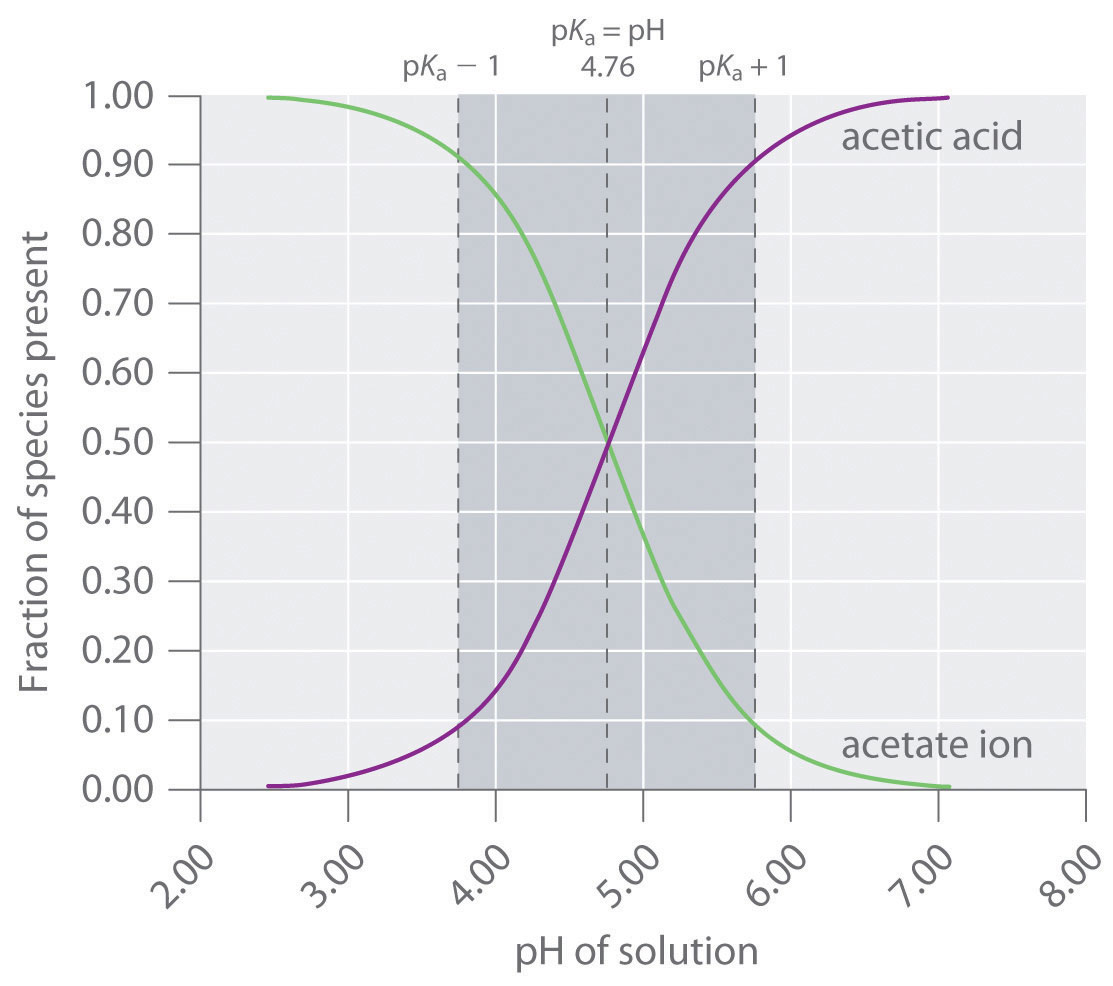
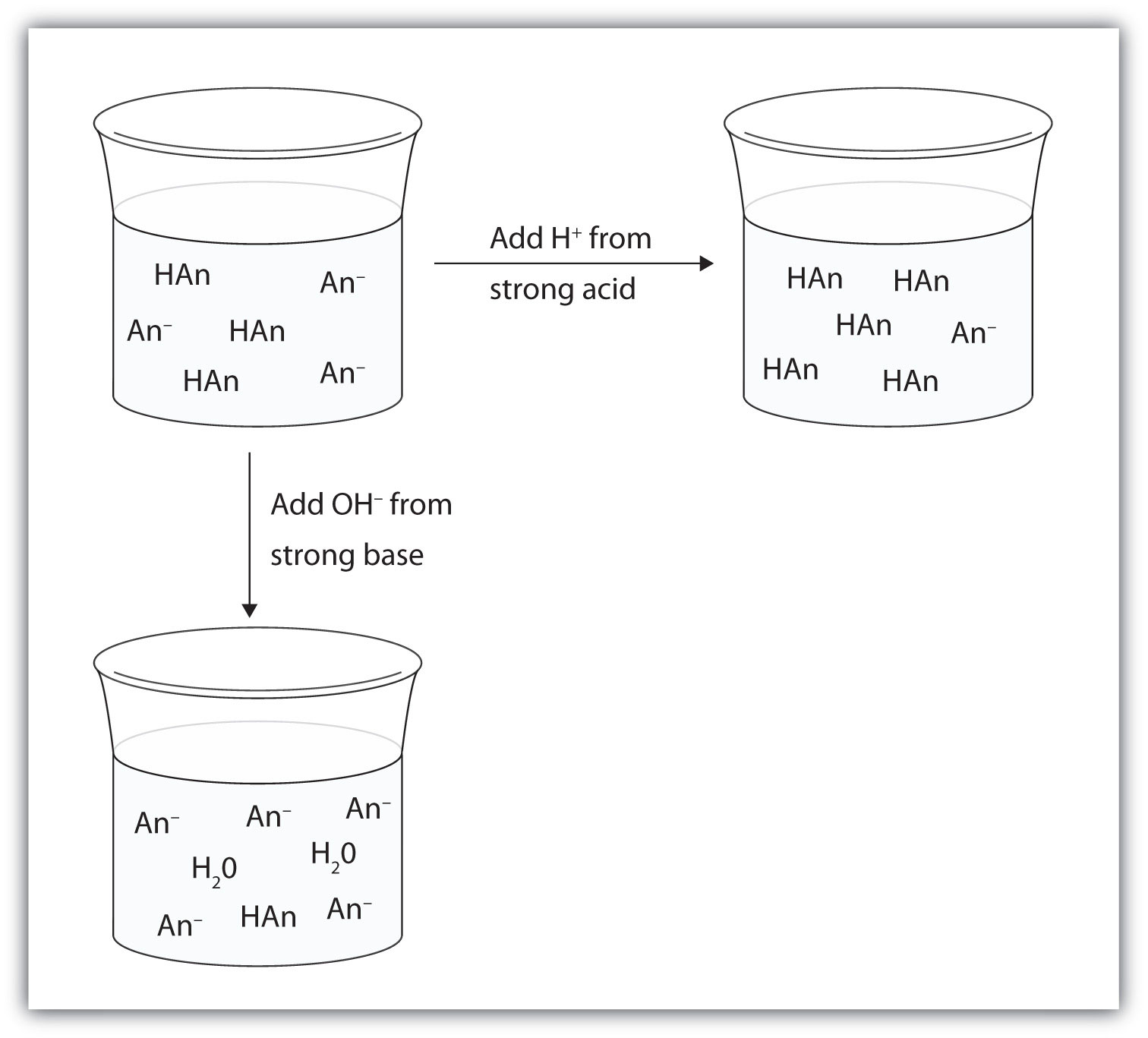
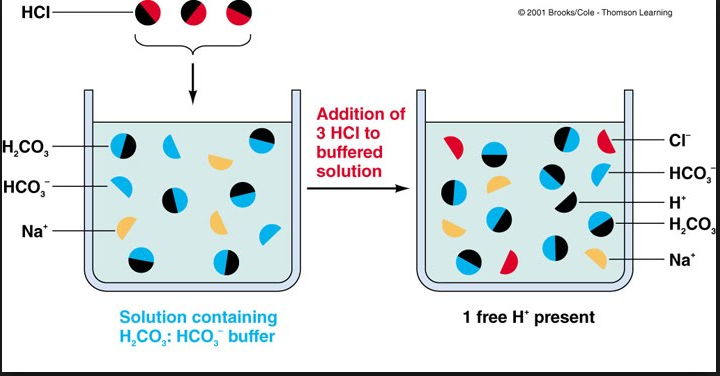
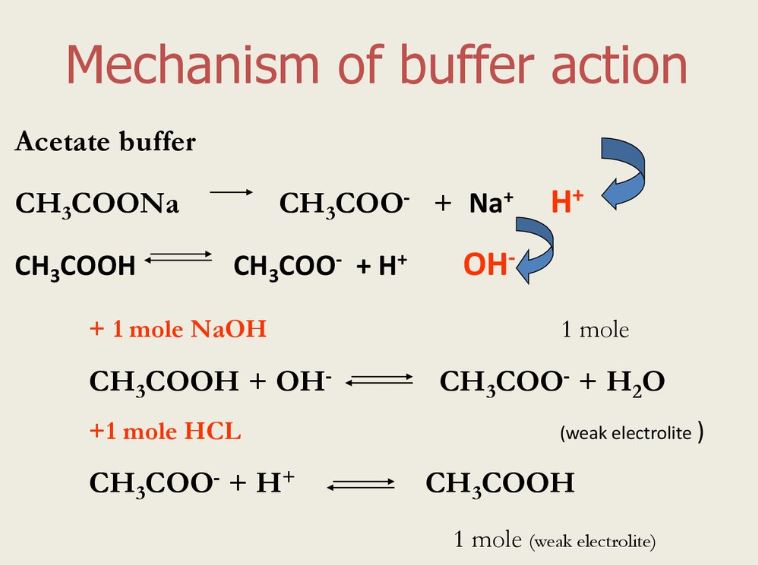
Compare 1 L of acetate buffer solution (0.50 mol of acetic acid and 0.50 mol sodium acetate) to 1 L of HCl solution Similarities

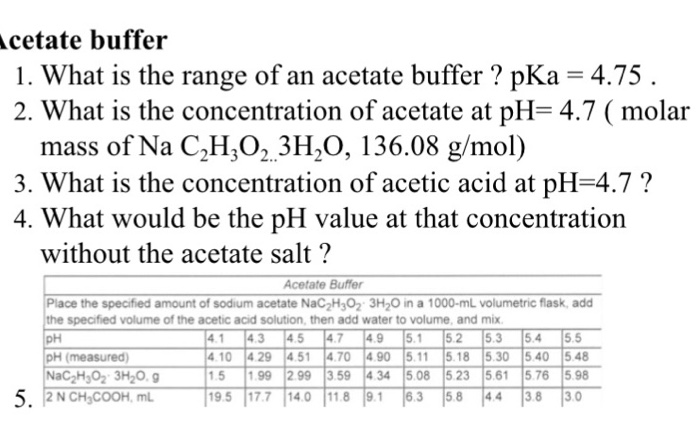

![BCH 312 [PRACTICAL] Preparation of Different Buffer Solutions. - ppt download BCH 312 [PRACTICAL] Preparation of Different Buffer Solutions. - ppt download](https://images.slideplayer.com/17/5267373/slides/slide_14.jpg)