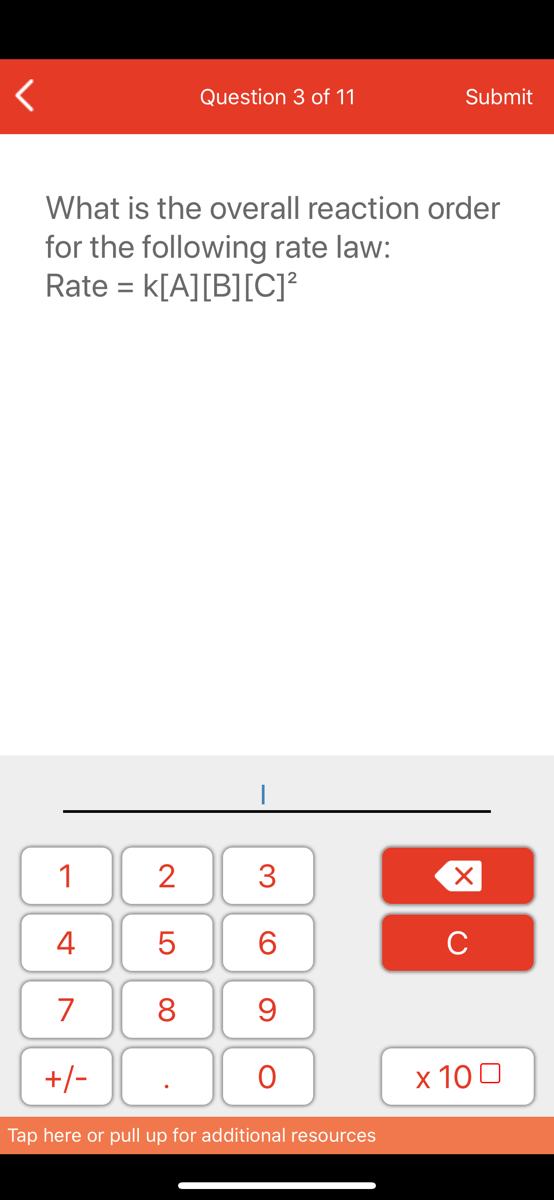
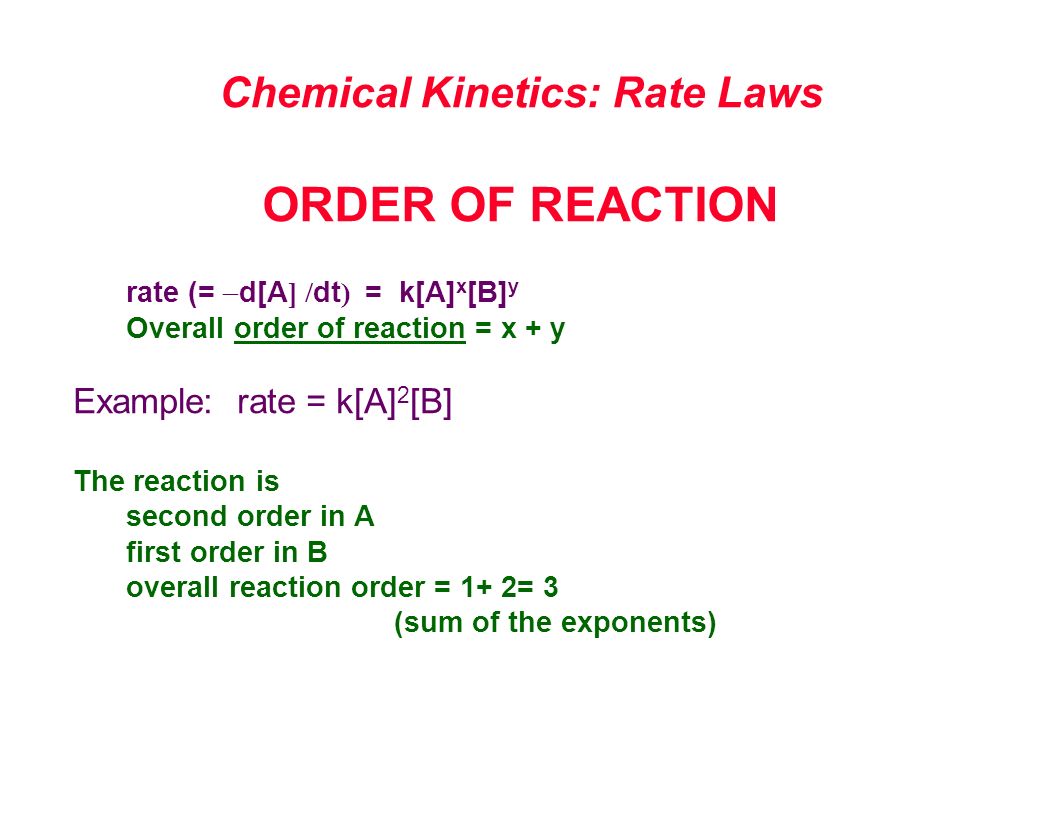
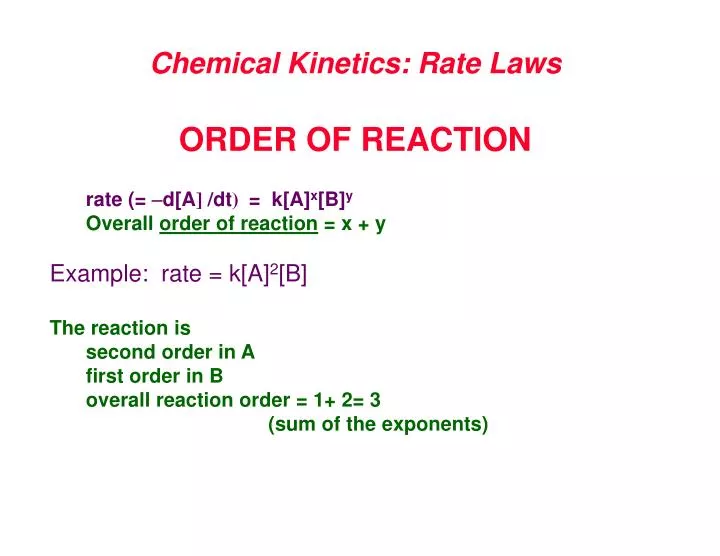
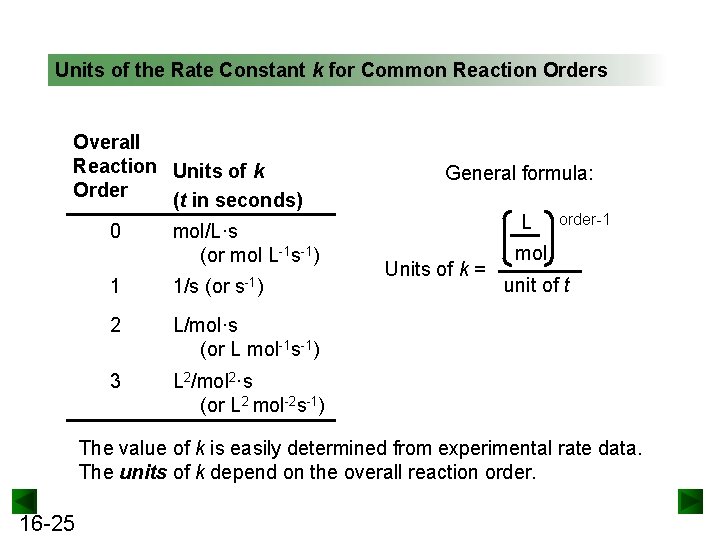
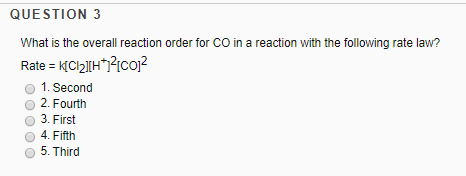
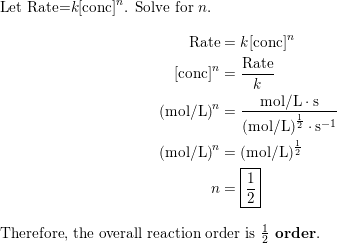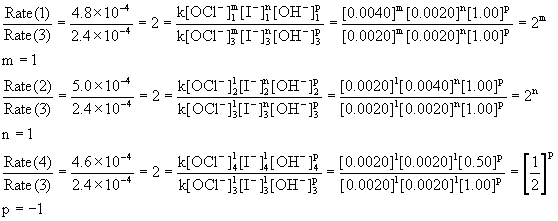The Rate Law. Objectives: To understand what a rate law is To determine the overall reaction order from a rate law CLE ppt download

How to Determine the Order of Reaction by Comparing Initial Rates of Reactions | Chemistry | Study.com
3. Short Answer = A reaction A + B - C obeys the following rate law: Rate = k (B)”. (a) If [A] is doubled, : [] how will - Answer Happy
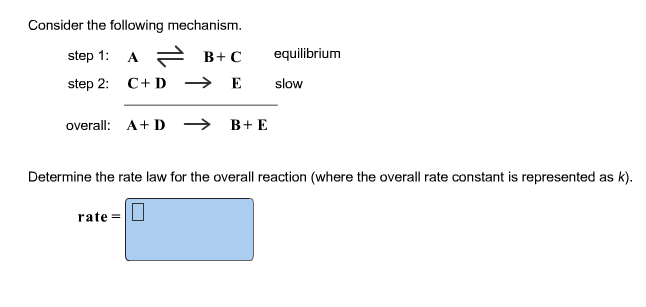
Determine the rate law for the overall reaction (where the overall rate constant is represented as k)? | Socratic
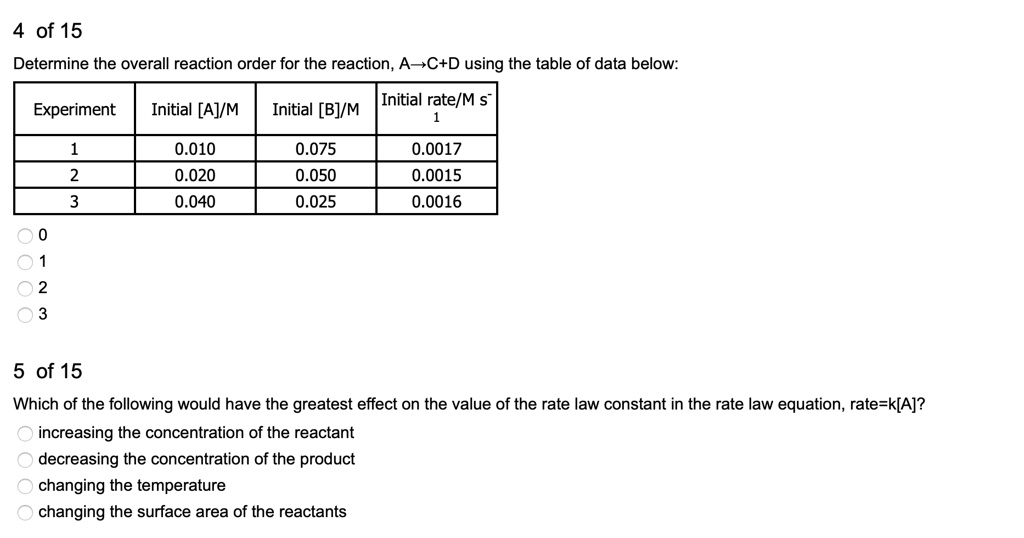
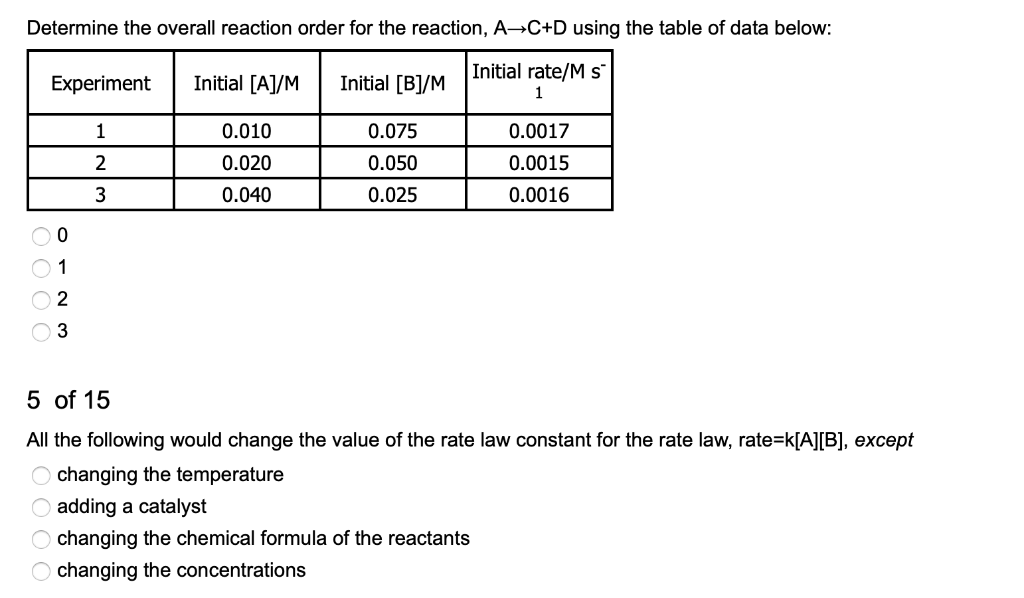
SOLVED:4 of 15 Determine the overall reaction order for the reaction, A C+D using the table of data below: Initial rateM s Initial [BJ/M Experiment Initial [AJIM 0.010 0.020 0.040 0.075 0.050

OneClass: Rate law and overall reaction order. I cannt figure out how to do this problem. I though I ...
![SOLVED:The reaction: A + 4 B + 3 C products is found to have the rate law: Rate k[A]- [B]l[C]2 Enter numerical values only. Enter 0 for zero-order; enter 1 for first-order; SOLVED:The reaction: A + 4 B + 3 C products is found to have the rate law: Rate k[A]- [B]l[C]2 Enter numerical values only. Enter 0 for zero-order; enter 1 for first-order;](https://cdn.numerade.com/ask_images/6ee2e0d6ba014ec6a717928c44a8650a.jpg)